Algis Kuliukas MSc (Human Evolution & Behaviour, UCL, 2001).
Paper submitted to Nutrition and Health, August 2002.
Abstract
The recent discovery of sahelanthropus tchadensis has brought into focus once more questions about the factors which may have led some ape clades to begin to evolve those most distinguished human traits: large brain size and bipedality.
This find, the oldest putative hominid yet, as well as recent nutritional studies have both strengthened the idea that human evolution occurred in water-side rather than arid, open savannah grassland habitats.
Evidence is accumulating that suggests that the large human brain is most likely to have evolved in littoral and estuarine habitats rich in naturally occurring essential fatty acids.
This paper adds further weight to this view, suggesting that another key human train, our bipedality, might also be best explained as an adaptation to a water-side niche.
Evidence from apes in the wild show that though preferring to keep dry, they do go into water when driven to do so by hunger and tend to do so bipedally. A new empirical study of captive bonobos found them to exhibit 2% or less bipedality on the ground or in trees but over 90% when wading in water to collect food.
A review of AL 288-1 (“Lucy”) skeletal morphology indicates a strong ability to abduct and adduct the femur. These traits, together with a very platypelloid pelvis, have never been adequately explained by traditional, terrestrial or arboreal models for early bipedalism but are consistent with those expected in an ape that adopted a specialist side-to-side ‘ice-skating’ or sideways wading mode. It is argued that this explanation of A. afarensis morphology is more parsimonious than others which have plainly failed to produce a consensus.
The paleo-habitats of the earliest known bipeds, and all the evidence reviewed here (as is that of the newly discovered Sahelanthropus), is consistent with the hypothesis that wading contributed to the adaptive pressure towards bipedality.
Word Count (Main Body Only) :17,674 (inc. tables 18,828, inc. charts 19,457)
Word Count (Total, inc. title, abstract, contents, acknowledgements & References): 21,810.
1. Introduction
The recent discovery of sahelanthropus tchadensis ? Buden et al (2002) has brought into focus once more questions of what factors may have led some ape clades to begin to evolve those most distinguished human traits: large brain size and bipedality.
From the paleoecology of the find it is rather clear that water was a significant part of his landscape. The fossils were found at the site of the ancient ‘Mega’ Lake Chad which dominated the area and the fauna found at the site included an abundance of an extinct group of ungulates – the anthacotheridea. These animals are considered to have been specialist amphibious animals, characterised as a kind of a ‘swamp pig’ (ref?) which are thought to be ancestral to the Hippopotomi. This clear potential association of the earliest hominid found with a water-side habit yet begs the question – what evidence is there that aquatic habitats generally might have driven the evolution of big brains and bipedality?
Recent nutritional and biochemical studies have strengthened the idea that human evolution probably occurred in water-side habitats and not, as is still largely advocated in many paleoanthropological circles, a predominantly semi-arid savannah environment.
Broadhurst et al (2002) show that there is a growing body of biochemical evidence suggesting that one of the key human traits, our large brain, could not have evolved on the savannah where, they argue, the quantity of certain naturally occurring essential fatty acids (EFAs) such as docosahexanoic acid (DHA) and arachidonic acid (AA) in the food chain is too meagre to have driven (or even allowed) such a dramatic increase in brain size. They argue that a water-side habitat, where such EFAs especially necessary for brain growth are very plentiful, is consequently more likely to have been the place where humans evolved.
One of the other key traits that distinguish humans from other apes is our bipedality. Since Darwin first suggested that humans and apes had evolved from a common ancestor there has been much speculation about the causative factors which might have led to the adoption of this unusual form of locomotion. Many of these models also involve food (e.g. carrying food, picking berries, peering over tall grass when hunting etc.) but almost always in the context of an assumed terrestrial or arboreal substrate and hardly ever in and around water.
Despite its simplicity, plausibility and consistency with evolutionary paradigms (Darwinian natural selection and the Crawford/Marsh notion of substrate driven evolution — ‘plastic heredity’) little serious scientific study has been undertaken about the plausibility that the first bipeds might have waded in water.
This paper offers some new empirical evidence from captive bonobos which supports the hypothesis that the earliest bipeds may have been wading apes and that the motivation for doing so might have been the search for food. Furthermore it argues that traits in the fossil record of the most well known of the early bipeds — Lucy — which have remained controversial despite over twenty-five years of intense scrutiny — is not only consistent with the hypothesis but actually resolves many of the dilemmas and contradictions which remain if moving through water is not considered as a possible mode of locomotion.
Finally it proposes that the paleoecology of the earliest hominids is entirely consistent with this water-side based model for the evolution of humans.
The idea that bipedalism was first practiced in wading apes is not a popular one. It was first published in an English-language journal by Sir Alister Hardy FRS (1960) but has received little attention since. At least twelve other models, based upon purely terrestrial or arboreal models, are widely discussed including... Interestingly, most assume that food was a major motivator for the new behaviour locomotion.
No true consensus has yet arisen amongst these models to explain the adoption of a bipedal mode of locomotion in hominids but one of the most promising lines of investigation in this area has been to try to find analogues of facultative bipedalism in extant apes. This approach was pioneered by Kevin Hunt who, in 1994, published a landmark paper about an extensive study of wild chimpanzees (Pan troglodytes) in their natural habitat in Gombe, Tanzania? The opening lines of his paper were…
In over 700 hours of observation time he found that the chimpanzees studied spent less than 3% of their time in a bipedal posture but that foraging for food in the lower branches of trees was the most common motivation for doing so, when they did.
Following in Hunt’s footsteps, Videan and McGrew (1999?) published findings from a similar study comparing chimpanzee bipedalism with their sister clade bonobos ( Pan paniscus) in captive settings. They found similar levels of bipedalism between the two and that food collecting was less of a motivator in captivity. ??
Neither study showed any evidence for wading bipedalism, however this was hardly surprising as Videan & McGrew’s study was conducted in enclosures that did not contain bodies of water and Hunt’s study of wild chimpanzees merely demonstrated how, in their typical habitat, chimpanzees usually avoid water completely.
The fact that chimpanzees do not spend much time in and around water today, however, does not mean the early ancestors of humans did not do so. Indeed it does not mean other apes today do not do so either. The questions remain: how do apes behave when they do move in water? And what evidence is there that hominid ancestors may have done so?
The next two section will attempt to answer one of these questions each.
2. Wading in extant apes
The extant apes Pan troglodytes, Pan paniscus, Gorilla and Pongo have not traditionally been associated with wet habitats and have rarely been observed wading in the wild. Indeed they have been considered so fearful of water that moats are often used to contain them in captivity. There is consequently a remarkable scarcity of scientific data about wading apes. A search of the literature revealed just how little has been written about the phenomenon. Even anecdotal information is rare although more recently, evidence (mainly film or photographic) has been accumulating suggesting that all of the Hominoidae may be more comfortable in water than might have been previously assumed.
Galdikas, Ellis and Sommer & Amman have all either commented about orang-utans (Pongo) wading or have published photographs showing such behaviours and Ashley Leiman of the Orangutan foundation made this statement “Since 1986 I have visited Tanjung Puting National Park in Indonesia, on numerous occasions. During this time I have frequently seen orangutans wading bipedally in the swamp and river.”
Gorillas have also not traditionally been linked with water but Ellis (1990:p57) provides anecdotal evidence in captive gorillas that they can swim. Also Doran & McNeilage (1998:p121) and Parnell (2001:p294), studying Western Lowland Gorillas in the field, provide evidence of splash displays and feeding in the marshy swamps of Mbeli Bai. Parnell (2000, personal communication) observed several bouts of bipedal wading in these animals and wrote….
Even chimpanzees, which have long been considered the most hydrophobic of all the apes, turn out to be surprisingly fearless in water when they are sufficiently driven by hunger to get their feet wet. Angus (1971:p51) and Nishida’s (1980) both provide anecdotal evidence of chimpanzee locomotion in water. In addition to this there has recently emerged some significant photographic footage of chimpanzees wading bipedally in fairly deep (chest high) water from a research student, Jess Tombs, working at a chimpanzee sanctuary in the Conkouati reserve lagoon (See Tutin et al. 2001).
Finally, in bonobos too (the least studied of the great apes) there seems to be growing evidence that they are less fearful of water and show a greater tendency to wade than their chimpanzee cousins. Uehara (1976), de Waal (1996:p185), de Waal & Lanting (1999:p79-82) all document anecdotal evidence of bonobos moving in water in the wild.
What is lacking in the literature, however, is a specific study into the way extant apes move in water. It is this gap in the knowledge base that this paper attempts to begin to fill.
2.2 Analysis of empirical data of captive Pan paniscus bipedality
Ten captive bonobos were studied at Planckendael wild-life park on the 12th April and 13th/14th June 2001. They have a sheltered enclosure which leads directly to a large island surrounded by a moat. Eight of the ten bonobos in the group were studied.
The principle behind the study and the methodology used was largely based upon Hunt's (1994) work with some changes.
The most obvious difference was that this study was with captive bonobos not wild chimpanzees. Secondly, the study focused on the substrate in which the bipedalism was observed rather than the behavioural context in which it occurred. In this regard it specifically undertook to identify and quantify the types of locomotion exhibited in water.
All observations were made via this method (a Sony digital handy cam with 25 frames/sec precision.) Over fours hours of continual bonobo behaviour was recorded for detailed, in some cases frame-by-frame, analysis later. Thus, in three days, it was possible to generate potentially 14,400 lines of, continuous second-by-second, data to analyse. (Although actually, because the technique allowed long periods of inactivity to be ‘skipped’, the actual number of data items recorded in the database was 1,319.)
Postural categories were based upon the work of Hunt (1994).
The most significant ones for this study were:
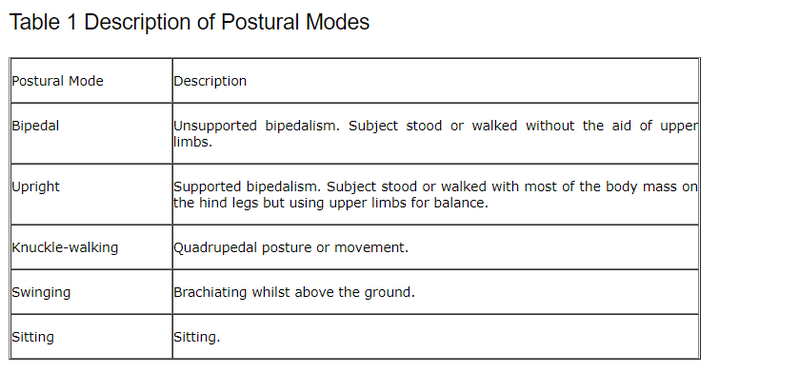
Inducing Wading BehaviourA mixture of observation techniques was used. Five focal studies were undertaken when one animal was followed continually for a half-hour slot. One hour's worth of observations were recorded of isolated, real or anticipated instances of contextual wading behaviour.
It is well known to staff at Planckendael that visitors to the bonobos often throw food items to them. This behaviour is strongly discouraged, but nonetheless the food is keenly accepted. Often these pieces of food fall short of their intended destination and, instead, drop into the large moat which surrounds it. When this happens the bonobos simply wade in and claim it.
One difficulty was that although it would undoubtedly have been very easy to induce the bonobos to wade into the moat, the authorities at Planckendael did not permit any such experimentation. Their reasoning was understandable: They did not want to be seen condoning the widely held practice of visitors throwing food to the animals. Also they are getting increasingly nervous about the prospect of the bonobos escaping the enclosure as they get more comfortable in the water.
This policy inevitably impacted on this study. Instead of controlling the wading events, the observations had to be predominantly reactive: waiting for and anticipating children (usually) to throw in pieces of food into the moat. This meant that the overall amount of time that bonobos were observed in water was very small (only about two minutes) however, workers at Planckendael have gone on record stating that their wading behaviour is very typical.
This study was primarily focused on the substrate that the bipedalism took place so four substrates were defined:
- Terrestrial, where the individual was at least partly touching land.
- Arboreal, where he or she was above the ground and not touching it. At Planckendael there are not many trees in the enclosure but there are a variety of climbing apparatus which were classified as arboreal nonetheless.
- «In Water», where no part of it was still touching dry ground.
- «Partly in water», where at least one part of the body was touching dry ground.
2.2.2 Results
As expected, analysis of focal study data (only) clearly showed that bonobos prefer the terrestrial substrate (72.63%), with arboreality taking up almost all the other time (27%). Only 0.37% of time was spent in contact with water.
Although the absolute percentages displayed here should not be taken too literally, there is no doubt that bonobos generally prefer not to enter the water.
The contextual data and focal data together were used to determine the levels of bipedality in different substrates.
Although only 37 seconds was spent in water with no contact with dry land at all, almost 92% of this time was spent in an upright (supported or unsupported bipedal) posture.
A larger proportion of time (121 seconds) was spent in water with some part of the body touching dry land. Apes in this substrate were upright for over 50% of the time. When terrestrial or arboreal, which accounted for over 99% of time for the group, the level of bipedality dropped to around or below 2%.
3.2.3 Discussion
The bonobos observed at Planckendael spent very little time in water and only did so at all because visitors threw food items (itself not encouraged) too weakly to reach them.
However there is no doubt that the amount of time they spent in the water was, very much, determined by human behaviour and it would seem that at least some individuals could have been induced into the water at will, in response to being offered food. Bonobos almost always entered the water feet first and adopted a bipedal posture even when the moat was shallow enough for them to have done so quadrupedally.
3.3 Conclusions
The anecdotal and observational evidence presented here clearly indicates that, although extant apes prefer not to get wet, they are more than prepared to do so if they are given a strong enough incentive to do so and that hunger is usually sufficient reason. In answer to the question ‘how do extant apes move in water?’ the answer would appear to be: bipedally. Or at least, it is far more likely to be bipedal than on land.
Equally clear is that in deep enough water, they have little choice but to move bipedally or swim. As only Gorilla out of the four great apes have been reported to be able to swim Ellis (1991:p55) it would seem that wading may occasionally be a life-saving behaviour in the wild.
It is difficult to imagine any other scenario with such a clear-cut, immediate survival benefit for moving bipedally as the one provided by waist deep water but it does beg a serious question: If a putative ancestor was regularly exposed to such a life-threatening habitat, wouldn’t they have been more likely, instead, to have evolved the ability to swim?
The next section investigates this problem and attempts to answer the question ‘what evidence is there that hominid ancestors may have waded bipedally?’
3. Was Australopithecus afarensis a wading ape?
In the 1970s Oxnard, (e.g. 1975) through the use of new techniques, such as multivariate, morphometric analyses, became sceptical of the prevailing view that placed Australopithecines ancestral to Homo. Using such techniques he demonstrated how their post-cranial skeletons differed so markedly from Homo as well as from the African apes that he had to conclude that they were probably of a different lineage altogether or, as he put it, (1983:p331) “that human bipedality was not the only experiment in this direction. The australopithecines are displaying for us another experiment, and, given that they are now extinct, one that failed.”
Oxnard agreed (p329) that Australopithecus, Homo, Pan and Gorilla are (or were) all closer to one another than any of them are to Pongo and that they are most similar to Homo in exhibiting a propensity for “a type of bipedality.” However he argued against the notion that it was an intermediate form of bipedalism, “close to the pathway of the evolution of bipedality as expressed in the evolution of man.”
Instead he suggested that Australopithecines in displaying uniqueness in their morphology may have been functionally unique too. He wrote (p 329) “They therefore displayed either a totally new and unknown manner of locomotion which would be unique in its own right (and which we will judge unlikely), or they possessed such a mixture of locomotor abilities, therefore anatomical adaptations, and therefore, in turn, bony morphologies, as to be rendered unique through being curious functional and morphological mosaics.”
It was not the objective of this study to concern itself with the phylogeny of the Australopithecines, although that is clearly of some relevance. The objective, in this section, is to question its curious morphology. Specifically, it is to consider Oxnard’s first option, the one he dismissed as unlikely, that it was the result of an unknown mode of locomotion, and simply ask: Was Australopithecus afarensis a wading ape?
We have seen that bipedal wading is not so unusual a mode of locomotion in the Hominoidae after all . All of them have demonstrated that they are capable and prepared to move in this way when necessary. It would seem likely, therefore, that their ancestors were also capable of the same but what actual evidence is there in the fossil record that this was the case?
Firstly it should be considered if the A. afarensis morphology has already been adequately explained in terms of its locomotor repertoire by existing models.
This will be tackled in three parts. In the first, the debate about the general locomotor repertoire of A. afarensis is discussed. In the second the attempts to relate her peculiar pelvic morphology to its potential functionality, and specifically her gait, are reviewed. Finally the third discusses the possibility that if A. afarensis did wade bipedally perhaps they had a way of doing so that was different to extant hominoids today.
3.1. In which ways did A. afarensis move: two modes or three?
Starting with the basics, the question must be asked: Was Lucy arboreal, terrestrial, a bit of both or what?
The debate thus far about the way A. afarensis moved (e.g. as summarised by Stern 2001) has been primarily focused on how arboreal she was and how human-like her undoubted bipedality manifested itself. The discussion seems to attempt to place Lucy on a linear scale with totally erect human-like, bipedality at one end and a totally arboreal climbing repertoire at the other. This essentially ‘bi-modal’ locomotory repertoire made sense because it naturally fitted the two substrates she was assumed to have moved in. She walked on the ground or climbed in the trees, the question was simply: how much in each?
Recently however, evidence of another mode to her locomotory repertoire may have been found that might complicate this picture. Richmond & Strait (2000) found notches on distal radii that were analogous to similar structures found in chimpanzees and gorillas associated with knuckle-walking. Their findings suggest that “bipedal hominids evolved from a knuckle-walking ancestor that was already terrestrial” (p 382).
They conclude that “Pre-bipedal locomotion is probably best characterised as a repertoire of terrestrial knuckle-walking, arboreal climbing and occasional suspensory activities, not unlike those observed in chimpanzees today”.
In the same journal Collard & Aiello (2000) review the finding and discuss the dilemma posed by it. On the one hand it could be argued that the knuckle-walking traits are “non-functional retentions from the common ancestor of hominoids and African apes,” “The alternative idea” they reason “that A. afarensis combined knuckle-walking, bipedalism and climbing — is somewhat counterintuitive, because it implies the use of two entirely different modes of terrestrial locomotion.”
The issue of phylogeny is fundamental to this particular debate. It is possible that A. afarensis evolved from a knuckle walking ancestor, even though it no longer did so itself, in which case it could be argued that the traits were just “evolutionary baggage” from the past. However that argument might be seen as a little convenient to some. The same paper found that A. africanus, a putative descendant of A. afarensis,had lost those traits. If they were phylogenetically related and if the knuckle-walking traits were evolutionary baggage in A. afarensis, why did they then disappear in A. africanus? Baggage is baggage.
Furthermore, recent research has indicated that inferring phylogeny from skeletal structures is more unreliable than had been considered in the past (Gibbs, Collard & Wood 2000), adding further weight to the argument that the traits were functional, and others have highlighted the plasticity of bone during lifetimes. Oxnard (1983: p97) said “There is now every good reason for believing that most biological materials, especially bone, must be considered as anisotropic, poroelastic, materials.”
To conclude: The most parsimonious explanation for the knuckle-walking traits of A. afarensis has to be, simply, that A. afarensis was a knuckle walker.
This is indeed counterintuitive if one assumes that A. afarensis moved only in the terrestrial and arboreal substrates. However if one considers that its bipedality was primarily for moving in water, then the dilemma disappears. Seen this way A. afarensis had three modes of locomotion for three different substrates: climbing and brachiating for the trees; knuckle-walking on solid ground and wading in water. It is claimed here that a clearer explanation has not, thus far, been suggested.
3.2 Predicting Lucy’s bipedal gait
Whether A. afarensis had two or three modes of locomotion nobody doubts that they were, at least in some way, bipedal.
The AL 288-1 post-cranial remains are remarkably complete, especially those structures associated with her bipedality. It is reasonable, therefore, that accurate inferences might be made into the way she moved and there have been no shortage of attempts to do so.
Not surprisingly too, there have been many excellent reviews of A. afarensis morphology. The reader is urged to refer to Aeillo & Dean (1999:Chs.14,19,20-21), which compares human, great ape and australopithecine bipedal morphology in a systematic and clear way.
Several elements of the post-cranial skeleton have been recognised as indicating her bipedality although, for brevity, only those concerned with the pelvis and femur (and not for instance vertebrae or feet) will be considered here.
Possible functional significance of the australopith femuro-pelvic complex
One of the most remarkable aspects of the A. afarensis pelvis is its pronounced iliac crest. A thorough morphometric study (Marchal 1999) comparing hominid pelvis morphologies concluded this was the clearest difference between them, noting that it was “very different from the human condition.” (p355).
This and other differences are reviewed by Aiello & Dean (1999:p451-453) where it was further observed that the A. afarensis pelvis further differs from the human condition “in their extreme width” not only at the iliac crests and at the acetabulae but also with a very platypelloid (flattened dorso-ventrally) pelvic inlet.
It has been difficult to explain convincing functional reasons for these features.
Aeillo & Dean (p 451) suggest “there are two current interpretations” and then go on to describe the morphological features that the proponents of human-like upright walking and the so-called “bent-hip, bent-knee gait” (from now on referred to as ‘BHBK’) use to back their arguments.
BHBK versus Fully Upright versus Wading
The first specimens (a piece of proximal tibia and a distal femur) to be retrieved from Hadar in Ethiopia in 1973 were observed by Owen C. Lovejoy and diagnosed as have belonged to a hominid “that could walk upright.” He based the diagnosis on its clearly valgus knee, which indicated to him that not only was she bipedal, but that she exhibited an essentially human-like (erect) form.
Lovejoy added further weight to this view later when AL 288-1 was analysed a couple of years later, arguing that the wide lateral flare of the pelvis indicated that they were used as abductors of the pelvis on an extended thigh, as with humans during walking. This view was the most logical interpretation especially after recent work (e.g. Patriaco 1981) had demonstrated, with myoelectric plots, the importance of the muscles involved with abduction in the human bipedal gait. Lovejoy consequently promoted an essentially human-like gait for A. afarensis even though its femuro-pelvic complex was quite different, morphologically.
Later, however, Stern & Susman (1983) stressed that other traits, such as curved phalanges, indicated a strong adaptation to arborealism. They suggested therefore that A. afarensis could not have been fully adapted to the kind of bipedalism we understand and suggested that instead it exhibited a more chimp-like, intermediate form, BHBK.
Further evidence in favour of BHBK was found by workers such as Tardieu (1991), Berge (1994) and Abitbol (1994).
After studying displacements of centre of gravity. Tardieu proposed a “rope-walker” gait model for chimpanzees and early bipeds suggesting that their low centre of gravity meant that their “bipedal dynamic equilibrium was still precarious.”
Berge performed biomechanical studies on A. afarensis reconstructions based on two different (one ape-like and one human-like) gluteus muscle arrangements. She concluded (p271) “The present results lead to the conclusion that the bipedalism of Australopithecus must have differed from that of Homo. Not only did Australopithecus have less ability to maintain hip and knee extension during the walk, but also probably moved the pelvis and lower limb differently.” She characterised it as a “sort of waddling gait” with large rotatory movements of the pelvis and shoulders around the vertebral column.
And Abitbol (1994) cast serious doubt on the notion that A. afarensis walked in a fully-upright manner by challenging the way that her hip joints have been reconstructed in models to date, in particular the assumption that the hip joints “are aligned in a vertical plane” (p211). He concluded (p225) “one may justifiably wonder if Lucy’s erect posture might not reflect some characteristics of nonhuman-like bipedal posture and locomotion.”
Those in favour of a fully-upright mode were bolstered recently however when the BHBK hypothesis was countered by Crompton et al. (1998). Through predictive dynamic computer modelling, they were able to suggest that BHBK would have been energetically very inefficient and would also have generated an excessive heat load to the individual. They conclude (1998 p 71) “It is thus difficult to envisage a selective pressure sufficient to bring about the undoubted bipedal adaptations of this species, if it habitually utilised ‘bent-hip-bent-knee’ bipedal walking.”
Stern (1999:p567) replied to this by suggesting that his colleges “never doubted that BHBK walking is more energetically costly” but that (p569) “the extra cost would have been small.” His last words on the matter were (p569) “It was only when life on the ground overwhelmed in selective importance any use of trees for feeding and escape, that the human ancestor was free to evolve the highly efficient mode of bipedalism with which we are personally familiar.”
The debate rages on today with no prospect of consensus in sight. A salvo from the trees, followed by a retort from the ground. Perhaps peace may be found by questioning their common assumption that the lifestyle of A. afarensis was not influenced by water.
Three positions on the bi-modal locomotor axis for A. afarensis are considered.
In the paper by Crompton et al. (1998), BHBK was suggested to have been energetically less efficient but this was based upon the unspoken assumption that her bipedality was purely terrestrial. Therefore no computer model was devised to test how BHBK would have performed in water. If they had, they might have come to a very different conclusion.
It would seem logical that, whilst wading, a significant amount of Lucy’s body weight would have been supported by inherent hydrostatic buoyancy, reducing the assumed (terrestrial) costs of maintaining that posture. This buoyancy could also explain the characteristically small, relative to body size, femoral head (Aiello & Dean 1999:p470-1) found in australopithecines which is normally interpreted as being correlated with the stress load resulting from body mass. Those volunteers who practiced BHBK for the waddling races in the swimming pool found it quite a comfortable, if unnatural, mode to adopt.
Their other main objection to BHBK, that it would have resulted in excessive heat generation, could also be easily countered if one assumed its bipedality was due to wading, simply because of the significant cooling effect of water.
Thus if A. afarensis was at least in part a wading ape Crompton et al’s published specific objections to the BHBK gait could be withdrawn.
BHBK Model
Ironically Berge (1994) used the same terrestrial assumption in arriving at the opposite conclusion. Her argument for BHBK or “waddling” was based largely on the belief that Lucy’s pelvic morphology would have made it difficult to maintain hip extension during walking and therefore that a fully-upright mode was unlikely. Again, she clearly did not consider if this would have also been the case in water. For the same reasons as above, it would seem logical that a totally human-like posture would have been far easier to maintain in water than on land for an early biped as long as it was deep enough.
Arboreal Model
The most extreme argument on the bi-modal terrestrial-arboreal axis, that A. afarensis did not spend much time on the ground at all and that it’s bipedality emerged from a purely arboreal niche does not make a clear distinction between the habitats of extant African apes such as Pan — the 6th (theoretical) test of the wading-origins model . If A. afarensis became bipedal in this habitat, why are chimpanzees not also bipedal today? Once again however, if her habitat is seen as being one where she practiced three modes of locomotion in three different substrates then one can clearly see why purely terrestrial/arboreal chimpanzees today would not be expected to be bipedal.
3.3 Sideways wading – Is it morphologically plausible?
This section began with the possibility that A. afarensis skeletal morphology might be so radically different from anything else yet found that perhaps Oxnard (1983:p329) had been right with his first (and most unlikely) explanation for it: That they had adopted “a totally new and unknown manner of locomotion which would be unique in its own right.”
Since then we have seen that a number of the controversies that surround the australopithecine locomotion debate might be solved if wading was considered as a possible mode for its bipedality. However none of this has, so far, gone any way to explaining its peculiar traits. Indeed earlier we saw how both extant apes and humans were able to wade satisfactorily without them.
The final part of this section proposes a potential functionality, based on a specialised mode of wading, which may explain them.
The 3rd prediction made of the wading-origins model was that any ape that became specialised to wading would optimise its wading method and in the human wading experiments we found a clear way how this might have been achieved. There was a clear correlation between speed (and drag) and submerged body profile in the water so, logically, through individual experimentation, cultural learning and/or natural selection a putative wading ancestor should adopt behaviours, and ultimately morphologies, that would minimise this profile.
The experiments which compared wading modes suggested that sideways wading, which resulted in a much smaller submerged profile, would theoretically be the fastest and most efficient if only human subjects could have propelled themselves sideways with sufficient force, which they evidently could not.
So we are left with an intriguing question: What evidence is there that A. afarensis was able to propel itself with sufficient force sideways to make this odd putative mode of locomotion a plausible one?
The muscles involved in such a sideways thrust are likely to be complex in humans and, of course, can never be known in extinct species of which only fossils remain. Those that took part in the sideways wading experiments in a swimming pool described the best way of doing this, after a little trial and error, as a kind of “kicking out” action to push away with the rear leg and alternately “reaching out and pulling back” with the front one. Whatever the specifics of the movement, it would seem that a large part of the force needed to propel someone sideways would come from those muscles involved with the abduction (moving out laterally away from the medial line) and adduction (pulling back closer to the medial) of the femur from the pelvis.
The most promising approach, then, might appear to be to investigate the specific musculature and associated skeletal structures of human hip abduction and adduction. This could then be compared with the known morphological differences between A. afarensis and humans to see if any biomechanical inferences can be made relating to its ability to wade sideways.
Firstly there will be a brief description of the most significant muscles that arise from the iliac crest and their general role in humans. This is followed by a list of related morphological differences in A. afarensis pelvis and femur. Finally an attempt is made to infer the kind of limb movements that would be enhanced by these differences.
Aeillo & Dean (1999:p410-413) describes the muscles and movements of the lower limb in humans.
The abductors are the Gluteus medius and Gluteus minimus.
Both arise from the iliac crest. The minimus lies underneath the medius and arises more from the centre of the iliac blade, towards the hip joint. (See Fig 22 below to clarify the relative positions of gluteus muscles in the great apes and humans. Taken from Aiello & Dean (1999:p414 after Sigmon (1974)
Both insert onto the femur, the gluteus minimus more posteriorly and the gluteus medius more anteriorly.
“The position of these muscles over the lateral side of the hip joint and their function as abductors and stabilizers of the pelvis are unique features of human bipedal locomotion.” Aeillo & Dean (1999:p413)
The Gluteus minimus and medius that are therefore likely to be the ones primarily concerned lifting the leading leg away and forward from the body and kicking out, and back, the rear leg in sideways wading.
There are five adductor muscles. The largest, Adductor magnus, arises from the ischial tuberosity and the ischiopubic ramus. It inserts on the femur along the linea aspera. Four of the others (pectineus, adductor brevis, adductor longus) fan out from the pelvis in similar ways and insert at points along the length of the femur. One, however, the gracilis inserts onto the tibia.
Applying basic lever theory to the biomechanics involved with hip abduction (for example) makes it clear that the efficiency of the force acting on the femur is related to the distance of from the hip socket from the insertion point onto the bone and the distance from the hip socket to the feet. (See fig 23 below).
Any combination of these would be predicted to improve the power and/or efficiency of the thrust of hip abduction but A. afarensis would appear to have all three.
According to Aiello & Dean (1999:p470) biomechanical neck length in australopithecines is long in comparison to humans and African apes (p461). However they say that this is more pronounced in the later, more robust australopithecines than in the earlier ones, such as AL 288-1 which are “closer to the modern human mean.” Berge (1994:p261) just describes the femoral neck as “very long.”
A. afarensis has been noted for their short limb length. Berge (1994:p261) describes “a very short femur” in AL 288-1 and recent studies (e.g. Kramer and Eck 2000) have even suggested that this might have been adaptive for slow, short-distant walking.
It is impossible to deduce anything about the thrust of Lucy’s gluteal muscles but a more prominently flared iliac blade from which they arose would seem to be consistent with the hypothesis that they were stronger.
According to Aiello & Dean’s summary of Lovejoy’s interpretation of the pelvis (1999:p451) “The length of the iliac blade, together with the corresponding length of the femoral neck, would give the muscles an even more favourable lever advantage in abduction than human [my emphasis] iliac-femoral morphology, and considerably reduce the force on the femoral head at the tip joint.”
Adduction forces could be treated similarly and would be increased if :
- The muscles described were more powerful,
- Leg length decreased or
- The distance between the acetabulum and the points of insertion on the ischiopubic ramus was increased.
The analysis can largely be repeated from that of the abductor muscles. But in addition Berge (1994:p267) notes that “it appears that the australopithecine adductor musculature must have been more powerful than that of humans” because of longer lever arms.
There is one more aspect of A. afarensis skeletal morphology which would be consistent with sideways wading model. A number of workers (e.g. Berge 1994:p261, Aiello & Dean 1999:p452, Rak 1991) have commentated on the platypelloid (wide laterally but thin dorso-ventrally) form of its pelvis. Aiello & Dean for example identify that the pelvic inlet sagittal diameter is 58% of the transverse diameter in A. afarensis compared with 78% in humans.
Rak (1991) explains the wide pelvis as an adaptation to reduce the vertical displacement of the centre of mass during terrestrial walking which, he argues (p286-7) “increases the vertical component of the ground reaction force, an increase that implies a less efficient forward progression, since more energy is expended to cover a given distance” and “the cyclic falling of the centre of mass causes an increase in joint reaction force and thus affects the joints and their physiology.”
He contends that the ancestral pre-bipedal hominoid essentially had a choice of two ways of solving this problem — either to widen the hips as did A. Afarensis or to lengthen the legs as did the ancestors of Homo. This is an ingenious idea but it is one that is, again, based upon the assumption that water was not a factor.
Rak (1991:p285) compared the pelves of A. afarensis with Homo by superimposing one image on another. He scaled the image of AL 288-1 so that they were the same height dorso-ventrally in order to emphasis Lucy’s relatively wide pelvis. This has become the traditional interpretation of A. afarensis morphology. However the diagram, and perhaps that view of her pelvis in general, is a little misleading because it requires scaling to make Lucy’s pelvis wider than it actually is.
If one ignores scaling and measures the pelvis itself one finds that it is not so much that her pelvis was wide laterally but that it was shallow dorso-ventrally. In fig. 25 (below) a photograph of pelves from both A. afarensis and H. sapiens together was scanned and measured using CorelDraw 8 software to estimate the relative widths of the pelvis both dorso-ventrally and laterally.
This shows that this human pelvis, at least, was wider than Lucy’s but was more than 50% deeper dorso-ventrally. The platypelloid shape of the A. afarensis pelvis might be its most remarkable feature.
Inferring the external body shape from the skeletal is not a precise science but this data would imply that the bodies of A. afarensis were platypelloid at the hip and presumably also lower abdomen. Rak (1991:p285) agreed with this point indirectly by arguing against the concept – put forward by McHenry 1986, Tague & Lovejoy 1986 and Lovejoy 1988 — that Lucy’s wide false pelvis was explained by the need for a biped to accommodate the abdominal viscera. Rak argued basically that the reduced anterior-posterior distance would reduce the capacity of the abdomen.
As the human experiments showed in the previous section, submerged body profile is significantly reduced when wading sideways and therefore, it would be logical that any ape specialising in such a niche might evolve a lower body shape that reduces this profile still further.
After consulting general body proportions from Zihlman (1978), AL 288 estimated stature from Aiello & Dean (1999 p 264) and AL 288-1 femur length from Zihlman (1978 p 89) it was decided that the Planckendael bonobo Hermien were similar enough in size and shape (frontally at least) to AL 288-1 to use her profile for a very simple comparison with humans.
Notice here that her sideways profile is hardly smaller (about 5% less) than her frontal profile. This is partly due, no doubt, to the imperfection in the frontal and sideways postures. Neither were as accurate as for the human images. It would seem, however, that bonobo sideways profile is rounder than that of humans.
It is predicted that, A. afarensis would differ significantly in this regard from P. paniscus. Perhaps their frontal submerged body area plot would be wider but their sideways profile is likely to be far less, (probably about half) evidenced largely by her platypelloid pelvis with its width ratio of over 2:1.
A plot of submerged frontal body profile against water depth shows that bonobos, due to their more ‘squatting’ posture, have a greater profile in shallower depths than humans indicating that A. afarensis might incur greater drag costs when wading frontally even than humans.
Unfortunately it was not possible to race bonobos in Amersham multi-pool but one peculiar incident was reported by the field worker Maarten De Rouck at Planckendael, which might give a clue as to how they might have performed if they could have been induced to do so.
He described an incident he observed recently thus: “Last week it was a rather rainy day and Kidogo and Hortense were sitting outside and when it started raining rather hard those two ran inside bipedally and they ran sideways toward the enclosure.” He also observed this on one other occasion. One might speculate about whether this behaviour has been reported elsewhere and, if so, about its functional significance.
Any suggestions about how A. afarensis might have performed in this experiment are even more speculative. However, it is suggested here that, considering Lucy’s remarkably shallow pelvis and her apparently highly adapted skeletal structures for abduction and adduction, she would have been able to wade very quickly indeed, especially if she did so sideways.
3.4 Conclusions
In this section several controversies and arguments that have arisen in interpreting the fossils of A. afarensis. A consensus view about them has clearly not emerged and so, it is argued here, that the morphology of AL 288-1 has not been adequately explained in terms of its locomotory repertoire by the non-wading models. Several of these points of contention have been shown to be resolvable if the assumption that water had no effect on the evolution of hominids is relaxed.
On the basis of these preliminary findings it would appear that A. afarensis would indeed have been able to propel itself with sufficient force and would have been sufficiently streamlined to make sideways wading a plausible mode of locomotion in waist deep water. This might seem a little fanciful at first but it is the logical conclusion of this line of enquiry. It is consistent with the fossil record and, it is suggested here, provides a clearer more parsimonious explanation of its morphology than any of the alternatives.
Therefore it is argued here that the 3rd prediction of the wading-origins model has been met by this test and much more fully than could have been anticipated.
Considering that A. afarensis fossils have been studied by so many experienced and respected paleoanthropologists, one is hesitant to question the assumptions they have based their work on for so long. The temptation is very much, instead, to follow in their footsteps. However, when one finds a plausible alternative assumption that seems to have been dismissed without being researched first, scientific curiosity insists that the possibility be explored.
Such an assumption appears to have been made about the habitat and lifestyle of the earliest hominids. The view that their time was spent only in trees or on dry land seems to have become de rigueur whilst the possibility that they were adapted to a water-side habitat has, as far as one can judge from published texts, simply been dismissed, although it is not clear on what basis this rejection has been made. We have seen that apes are at their most predictably bipedal when wading in waist deep water and it is clear that habitats where periodic wading could have evolved are common throughout the world both today and in the past.
4. Paleoecological and Geographical Evidence
Every fossil gives a reliable location for where its one previous owner died, a good estimate of when it lived and a fairly good idea of the paleo-climate and paleo-ecology of their habitat. If sufficient evidence can be extracted from what fragments are found to infer that they were predominantly bipedal then the other data could still be more than enough to refute the 4th prediction of wading origins model. All that would be needed was to demonstrate that the earliest bipeds lived in habitats where wading could not have been practiced.
4.1 Paleo-habitat of the earliest bipeds
In the following section the sites in which the earliest fossils of putative bipedal hominid have been found are reviewed.
6.2.1 Sahelanthropus tchadensis
The most recent hominid find at the time of writing is also the oldest: Sahelanthropus tchadensis. Although no post-cranial remains have been found, the anterior position of the foramen magnum indicates that it adopted a more upright posture and had a greater tendency towards bipedalism than modern chimpanzees do. Its age makes it a very interesting finding. It is old enough to possibly predate the last common ancestor of Pan and Homo. Dated (by comparative faunal remains only) at between 7 and 10 million years it lived well within the assumed range of dates for the mrca.
6.2.1 Orrorin tugenensis
The recent discovery of Orrorin Tugenensis from the Lukeino formation in Kenya at the end of 2000 (Senut & Pickford 2001) is the second oldest putative hominid yet found. It is almost old enough to question the long held assumption that bipedality evolved only on the hominid line since the Pan/Homo split. In fact before December 2000 most authorities were firming up on the idea that the mrca of Pan/Homo lived around 5.5 mya. If anything the tendency was to make the date even more recent. A paper by Baird et al.(2000), for instance, quoted three separate sources, including Takahata & Satta (1997), that had calculated the date as recent as 4.5 mya. Very few (e.g. (Kleindienst (1975), Goodman (1982: 260), Gribbin & Cherfas (1983), Hasegawa et al.(1985) and Edelstein (1987)) in the field have predicted that the mrca was already bipedal and that Pan and/or Gorilla had since reverted to quadrupedalism.
It is true that dates based upon the molecular clock had always been calculated with the usual confidence limits and so it would be reasonable to suggest that the date of last common ancestor had always been more blurred than some had assumed. Aeillo & Collard (2001) reminded us of this when they were the first to publish a specific reaction to the finding in March 2001. They state that “ Orrorin’sage falls within the molecularly determined range of the last common ancestor between humans and the African apes (8- 5 million years ago.)” They do not “seriously question” the accuracy of the age of the fossils but they do have doubts about the claim of Senut et al. that Orrorin was bipedal suggesting that it is “based on detailed aspects of the anatomy of the upper part of the thigh bone that are open to alternative explanations.” They also cast doubts about the legitimacy of placing Orrorin as ancestral to Homo (via a novel intermediary genus Praeanthropus .) So, for the time being at least, the exact status of Orrorin tugenensis is still in some doubt.
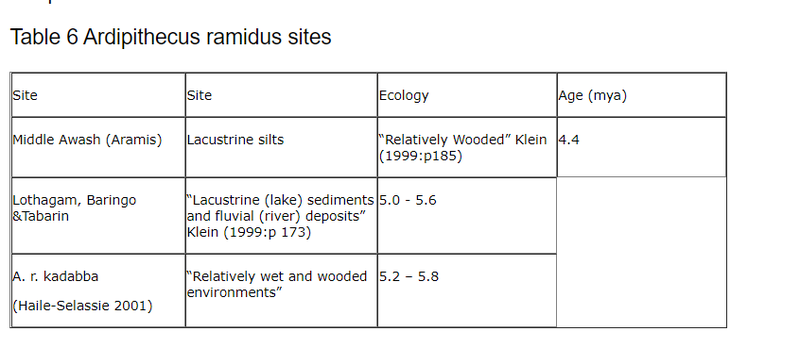
Before the discovery of Orrorin this was the oldest putative biped. This table shows the sites where A. ramidus has been identified. Klein (1999:p 188)6.2.2 Ardipithecus ramidus.
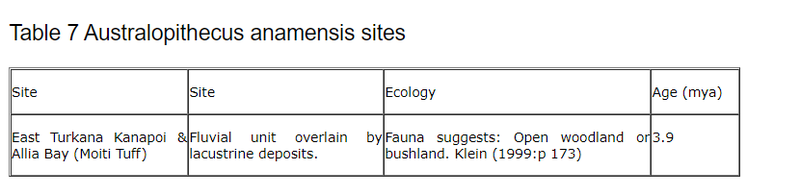
6.2.3 Australopithecus anamensis
According to Klein (1999:p177) A. anamensis is “the second oldest hominid species and the oldest for which bipedalism has been conclusively shown.” The fossil sites where it has been identified are shown in the table below from (p188).
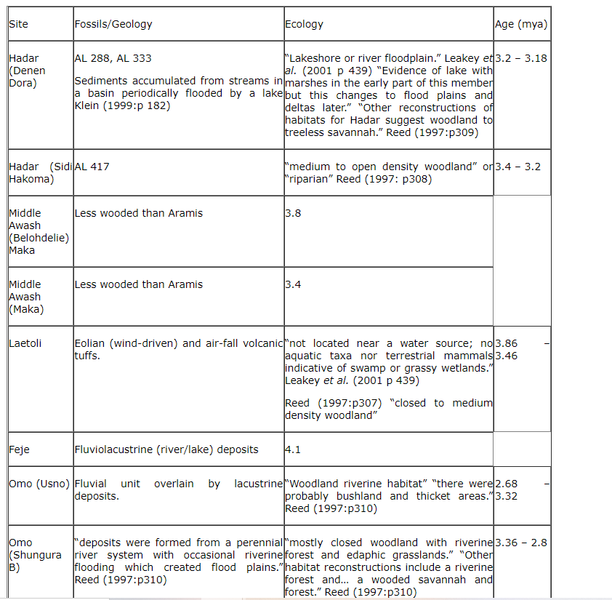
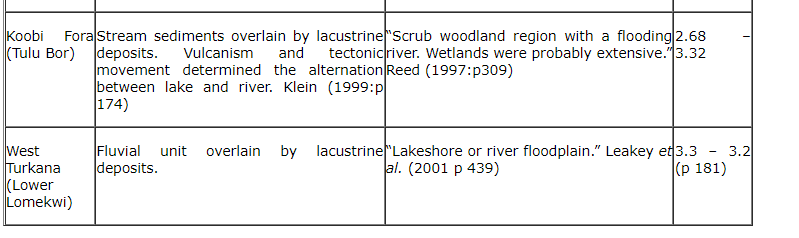
6.2.4 Australopithecus afarensis
The most complete hypodigms of the early bipeds. This table shows the earliest members in sites where A. afarensis has been identified. (Klein 1999: p 188)
Table 8 Australopithecus afarensis sites
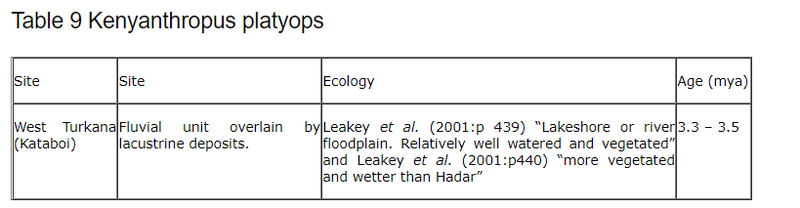
6.2.5 Kenyanthropus platyops
The most recent new hominid find. This table shows the sites where K. platyops has been identified. Data from Leakey et al. (2001.)




Bibliography








Image Upload
Add image